INTEGRATIVE SYSTEMS BIOLOGY FOR BIOMARKER DISCOVERY ACROSS DISEASES
This blog explores a systems biology framework for biomarker discovery across complex diseases. It highlights how multi-omics data, protein interaction networks, and AI-driven analytics can be integrated to identify and prioritize clinically relevant biomarkers. Key methodologies include differential expression analysis, hub protein detection, and multi-omics integration—advancing the path from molecular data to actionable diagnostics and therapeutics.

In the era of precision medicine, identifying disease biomarkers requires more than traditional gene-by-gene or protein-by-protein studies. Modern diseases are not caused by single molecular events but by complex, interdependent disruptions across biological systems. Whether the focus is cancer, neurodegeneration, cardiovascular disorders, autoimmune syndromes, or infectious diseases, biomarker discovery has evolved into a multi-layered, systems biology challenge.
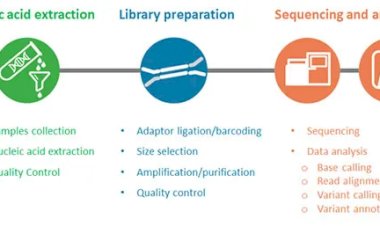
This transformation is driven by the explosion of high-throughput technologies—next-generation sequencing, mass spectrometry, single-cell omics, and spatial transcriptomics—which collectively generate vast amounts of molecular data spanning the genome, transcriptome, proteome, and metabolome. However, this data deluge is only as useful as our ability to synthesize it meaningfully.
To meet this challenge, researchers are integrating multi-omics datasets, network biology, and machine learning frameworks. These approaches shift the focus from isolated molecular signatures to functional networks, uncovering disease-associated pathways, key regulatory nodes, and latent biomarker patterns that would be invisible to reductionist methods. Importantly, these insights are no longer restricted to research contexts; they are increasingly translated into clinical applications, driving early diagnosis, prognostic modeling, patient stratification, and targeted therapy.
This convergence of disciplines marks a paradigm shift in biomedical research and clinical strategy—from reactive treatment based on symptoms to proactive intervention based on systems-level molecular understanding. In this integrative landscape, biomarkers are not merely indicators of disease but become predictive instruments, therapeutic targets, and guides for personalized care.
1. The Biomarker Landscape: From Thousands to the Few
Transcriptomic, proteomic, and metabolomic studies routinely identify hundreds to thousands of differentially expressed molecules. These candidates reflect the complexity of disease—pointing to disrupted pathways such as:
- Inflammation
- Oxidative stress
- Metabolic imbalance
- Protein misfolding
- Immune dysregulation
The biological diversity of these signals demands robust filtering techniques. High-throughput screens using patient-derived organoids and 3D cell cultures complement in vivo validation by recapitulating disease microenvironments. These platforms are especially powerful in cancer and neurodegenerative research, where context-specific responses matter.
Model organisms such as C. elegans, zebrafish, mice, and yeast help validate these molecular changes in a functional context. Their genetic and physiological parallels with humans—especially when combined with evolutionary conservation analyses—help filter out irrelevant noise and highlight biologically conserved markers.
Up- and down-regulated molecules offer different insights:
- Upregulated markers may suggest adaptive responses, stress signals, or hyperactive pathways.
- Downregulated markers often reflect loss of function, damage, or impaired cellular signaling.
This stage serves not only to reduce dimensionality but also to preserve mechanistic relevance, laying the groundwork for network-based prioritization.
2. Network Analysis: Zeroing In on Functional Hubs
Molecular entities do not function in isolation; they exist in a highly dynamic interactome. Once initial candidates are identified, researchers build protein–protein interaction (PPI) networks using tools such as STRING, Cytoscape, or GeneMANIA. These networks visualize how molecules orchestrate biological functions and disease phenotypes as part of complex regulatory modules.
Network topology analyses reveal:
- Degree centrality: how many connections a node (protein) has
- Betweenness centrality: how often a node bridges others in the network
- Clustering coefficient: how tightly a protein’s neighbors are connected
Algorithms like cytoHubba, MCODE, and PageRank are used to pinpoint hub proteins—critical nodes that serve as control points within the biological network. These proteins often act as master regulators or signal integrators, meaning perturbation at these nodes can shift entire biological states. Hub-based biomarker selection thus prioritizes functionally influential and disease-relevant molecules rather than those that are merely differentially expressed.
Increasingly, researchers are incorporating time-series data to model how these networks evolve during disease progression, adding a temporal dimension to biomarker discovery.
3. AI-Driven Biomarker Prioritization
With data volumes growing exponentially, artificial intelligence (AI) is indispensable. Machine learning algorithms—especially when trained on integrated omics and clinical datasets—can transform noise-laden biological signals into clear, actionable insights.
Key capabilities include:
- Classification: Support Vector Machines (SVMs), logistic regression, and neural networks distinguish disease states.
- Feature selection: Methods like LASSO, Random Forests, and SHAP isolate the most predictive biomarkers.
- Representation learning: Deep learning models like autoencoders, transformers, and graph neural networks extract latent biological signatures from high-dimensional inputs.
These models can identify non-obvious patterns that escape human intuition, especially when working with complex, nonlinear interactions. Additionally, AI enables multi-modal integration—combining molecular data with imaging, electronic health records, or wearable device outputs.
Importantly, explainable AI (XAI) is gaining traction, allowing clinicians to understand why a particular biomarker was selected—an essential factor for regulatory approval and clinical trust.
4. Multi-Omics Integration: A 360° View of Disease
Diseases are multifactorial and span genomic, epigenomic, transcriptomic, proteomic, and metabolomic layers. Single-omic studies often miss the forest for the trees. Multi-omics integration offers a systems-level understanding by aligning and interpreting diverse data streams.
Data integration frameworks such as:
- MOFA+ (Multi-Omics Factor Analysis)
- iCluster
- DIABLO (Data Integration Analysis for Biomarker discovery using Latent variable approaches for Omics studies)
- MixOmics
…use dimensionality reduction, latent variable modeling, or Bayesian approaches to identify cross-validated, multi-omic biomarker panels. These integrated models are robust against noise, consistent across populations, and capable of predicting disease subtypes or progression risk.
Increasingly, single-cell multi-omics (e.g., CITE-seq, scATAC-seq, spatial transcriptomics) is revealing cell-type-specific biomarkers, bridging molecular signatures with tissue architecture and heterogeneity.
5. From Discovery to Clinical Translation
Transitioning a biomarker from bench to bedside requires multiple validation layers and multidisciplinary collaboration.
- Analytical validation ensures precision, sensitivity, and reproducibility using techniques like qPCR, ELISA, or mass spectrometry.
- Biological validation uses cohorts, in vivo models, and functional assays to confirm biomarker relevance.
- Clinical validation correlates biomarkers with clinical outcomes, drug response, or patient stratification in real-world or trial data.
- Regulatory readiness addresses ethical issues, patient consent, and standard operating procedures to meet FDA or EMA guidelines.
Emerging technologies are bridging the lab-clinic divide:
- Liquid biopsies enable non-invasive biomarker sampling from blood, saliva, or urine.
- Single-cell analytics improve resolution for rare-cell populations, like circulating tumor cells or senescent immune cells.
- Digital pathology & AI integrate histopathological imaging with molecular data, enhancing diagnostic accuracy.
These innovations facilitate early diagnosis, risk scoring, and treatment tailoring, forming the backbone of precision medicine.
Applied Training Spotlight: IBRI Noida’s Hands-On Proteomic Analysis Program
Institutes like the Indian Biological Sciences and Research Institute (IBRI), Noida are playing a pivotal role in translating systems biology into real-world skillsets. IBRI offers hands-on training programs in cutting-edge proteomic analysis, enabling researchers and students to gain practical experience with protein quantification, interaction mapping, and data interpretation. These programs not only equip participants with the technical proficiency required for biomarker discovery, but also bridge the gap between academic learning and industrial applications in biotechnology and pharmaceutical R&D.
Conclusion: Toward Smarter, Faster, Broader Discovery
Today’s biomarker discovery is no longer confined to isolated experiments—it is a multidisciplinary, data-intensive, and clinically aligned process. By fusing systems biology, AI, and multi-omics, we can uncover molecular signals that are contextual, causal, and clinically actionable.
This approach holds the promise of early detection, patient-specific therapy, and proactive disease management. As computational tools advance and biological data become richer and more integrated, the next generation of biomarkers will be smarter, faster to validate, and broadly applicable across disease systems.
The future of diagnostics lies not just in identifying signals, but in understanding systems—making systems biology the central axis of 21st-century medicine.